Solanaceae
Overview
| Family | Solanaceae |
| Genus | 98 (16 assembled genus) |
| Species | 2700 (116 assembled species) |
| SI type | Type-1 |
| SI genes | S-RNase (pistil specific expression); SLFs (pollen specific expression) |
Organism Image

Description
The Solanaceae (/ˌsɒləˈneɪsi.iː, -ˌaɪ/), or the nightshades, are a family of flowering plants that ranges from annual and perennial herbs to vines, lianas, epiphytes, shrubs, and trees, and includes a number of agricultural crops, medicinal plants, spices, weeds, and ornamentals. Many members of the family contain potent alkaloids, and some are highly toxic, but many—including tomatoes, potatoes, eggplant, bell and chili peppers—are used as food. The family belongs to the order Solanales, in the asterid group and class Magnoliopsida (dicotyledons). The Solanaceae consists of about 98 genera and some 2,700 species, with a great diversity of habitats, morphology and ecology.
The name Solanaceae derives from the genus Solanum. The etymology of the Latin word is unclear. The name may come from a perceived resemblance of certain solanaceous flowers to the sun and its rays. At least one species of Solanum is known as the "sunberry". Alternatively, the name could originate from the Latin verb solare, meaning "to soothe", presumably referring to the soothing pharmacological properties of some of the psychoactive species of the family.
This family has a worldwide distribution, being present on all continents except Antarctica. The greatest diversity in species is found in South America and Central America. In 2017, scientists reported on their discovery and analysis of a fossil species belonging to the living genus Physalis, Physalis infinemundi, found in the Patagonian region of Argentina, dated to 52 million years ago. The finding has pushed back the earliest appearance of the plant family Solanaceae.
The Solanaceae family includes a number of commonly collected or cultivated species. The most economically important genus of the family is Solanum, which contains the potato (S. tuberosum, in fact, another common name of the family is the "potato family"), the tomato (S. lycopersicum), and the eggplant or aubergine (S. melongena). Another important genus, Capsicum, produces both chili peppers and bell peppers.
The genus Physalis produces the so-called groundcherries, as well as the tomatillo (Physalis philadelphica) and Physalis peruviana (Cape gooseberry). Alkekengi officinarum (Chinese Lantern) was previously included in the genus Physalis (as Physalis alkekengi), until molecular and genetic evidence placed it as the type species of a new genus. The genus Lycium contains the boxthorns and the goji berry, Lycium barbarum. Nicotiana contains, among other species, tobacco. Some other important members of Solanaceae include a number of ornamental plants such as Petunia, Browallia, and Lycianthes, and sources of psychoactive alkaloids, Datura, Mandragora (mandrake), and Atropa belladonna (deadly nightshade). Certain species are widely known for their medicinal uses, their psychotropic effects, or for being poisonous.
Most of the economically important genera are contained in the subfamily Solanoideae, with the exceptions of tobacco (Nicotiana tabacum, Nicotianoideae) and petunia (Petunia × hybrida, Petunioideae).
Many of the Solanaceae, such as tobacco and petunia, are used as model organisms in the investigation of fundamental biological questions at the cellular, molecular, and genetic levels.
SI type
The system with the broadest taxonomic distribution, which we term type-1 SI, is gametophytic and based on linked pistil S S-RNase and pollen S S-locus F-box (SLF)/S-haplotype-specific F-box (SFB). So far, type-1 SI has been found in four eudicot families: Solanaceae, Plantaginaceae, Rosaceae, and Rutaceae. After pollination, both S1- and S2-RNases can enter pollen tubes and be recognized by S1- or S3-SLFs, but only SCFS3-SLF complexes can ubiquitinate these S-RNases leading to their degradation by 26S proteasome, with the survived S1-RNase in S1 pollen tubes forming the S-RNase condensates (SRCs) resulting in self-pollen inhibition.
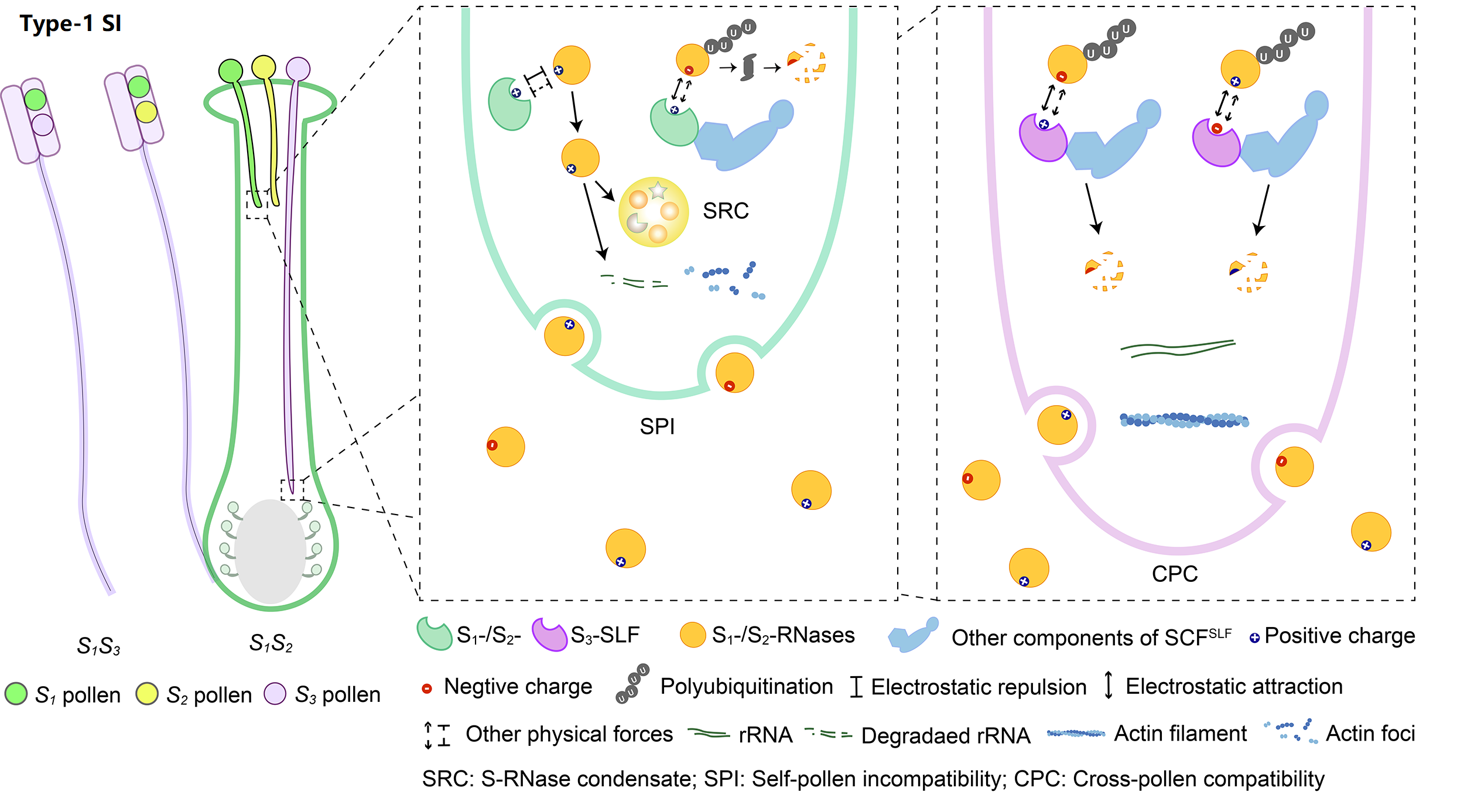
SI genes
Publication
Anderson, M., Cornish, E., Mau, SL. et al. Cloning of cDNA for a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Nature 321, 38–44 (1986). https://doi.org/10.1038/321038a0.
Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, McCubbin AG, Huang S, Kao TH. Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature. 2004 May 20;429(6989):302-5. doi: 10.1038/nature02523.
Kubo K, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima S, Ando T, Isogai A, Kao TH, Takayama S. Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science. 2010 Nov 5;330(6005):796-9. doi: 10.1126/science.1195243.
Liu W, Fan J, Li J, Song Y, Li Q, Zhang Y, Xue Y. SCF(SLF)-mediated cytosolic degradation of S-RNase is required for cross-pollen compatibility in S-RNase-based self-incompatibility in Petunia hybrida. Front Genet. 2014 Jul 22;5:228. doi: 10.3389/fgene.2014.00228.
Li J, Zhang Y, Song Y, Zhang H, Fan J, Li Q, Zhang D, Xue Y. Electrostatic potentials of the S-locus F-box proteins contribute to the pollen S specificity in self-incompatibility in Petunia hybrida. Plant J. 2017 Jan;89(1):45-57. doi: 10.1111/tpj.13318.
Zhao H, Song Y, Li J, Zhang Y, Huang H, Li Q, Zhang Y, Xue Y. Primary restriction of S-RNase cytotoxicity by a stepwise ubiquitination and degradation pathway in Petunia hybrida. New Phytol. 2021 Aug;231(3):1249-1264. doi: 10.1111/nph.17438.
Zhao H, Zhang Y, Zhang H, Song Y, Zhao F, Zhang Y, Zhu S, Zhang H, Zhou Z, Guo H, Li M, Li J, Gao Q, Han Q, Huang H, Copsey L, Li Q, Chen H, Coen E, Zhang Y, Xue Y. Origin, loss, and regain of self-incompatibility in angiosperms. Plant Cell. 2022 Jan 20;34(1):579-596. doi: 10.1093/plcell/koab266.
Tian H, Zhang H, Huang H, Zhang Y, Xue Y. Phase separation of S-RNase promotes self-incompatibility in Petunia hybrida. J Integr Plant Biol. 2024 May;66(5):986-1006. doi: 10.1111/jipb.13584.
de Nettancourt D (2001) Incompatibility and Incongruity in Wild and Cultivated Plants. Springer, Berlin Heidelberg, Germany. https://link.springer.com/book/10.1007/978-3-662-04502-2https://link.springer.com/book/10.1007/978-3-662-04502-2
Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467-89. doi: 10.1146/annurev.arplant.56.032604.144249.
Zhang Y, Zhao Z, Xue Y. Roles of proteolysis in plant self-incompatibility. Annu Rev Plant Biol. 2009;60:21-42. doi: 10.1146/annurev.arplant.043008.092108.
Iwano M, Takayama S. Self/non-self discrimination in angiosperm self-incompatibility. Curr Opin Plant Biol. 2012 Feb;15(1):78-83. doi: 10.1016/j.pbi.2011.09.003.
Fujii S, Kubo K, Takayama S. Non-self- and self-recognition models in plant self-incompatibility. Nat Plants. 2016 Sep 6;2(9):16130. doi: 10.1038/nplants.2016.130.